Upcoming pivotal phase 3 clinical trial for EB: Rheacell's systemic stem cell therapy
Rheacell new clinical trial phase 3
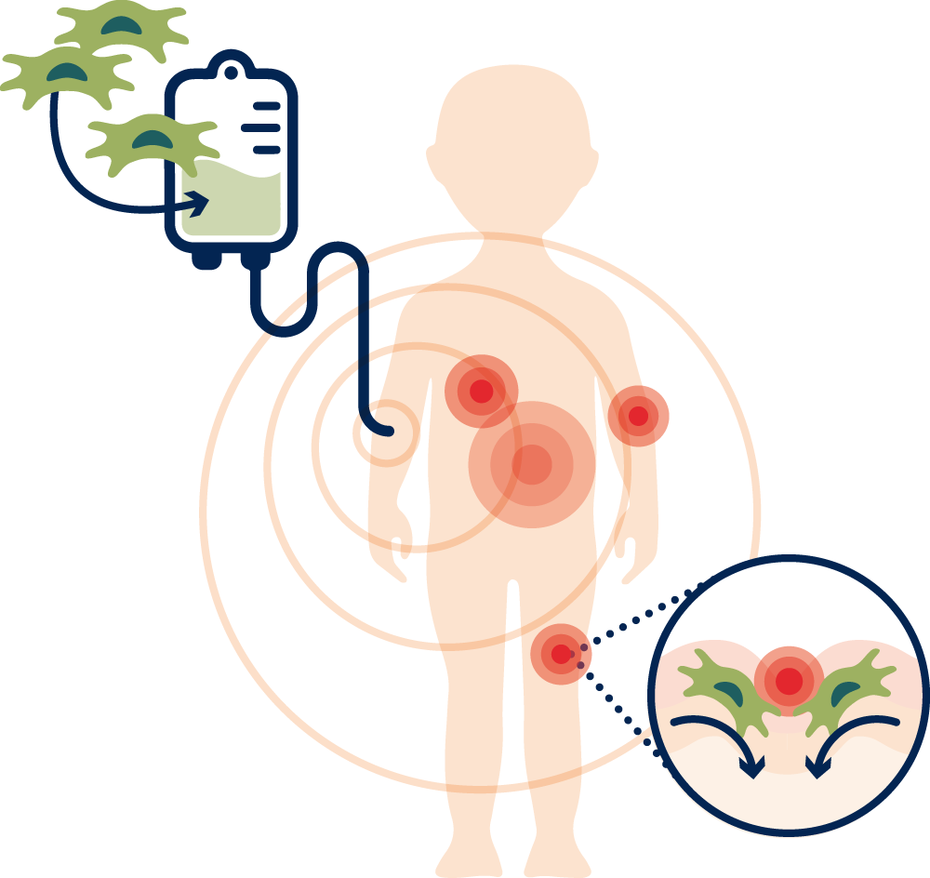
Due to their immunomodulatory and anti-inflammatory properties, ABCB5+ mesenchymal stem cells can offer a new innovative therapeutic approach for EB patients.
ABCB5+ MSCs have anti-inflammatory potential by interacting with surrounding immune cells and initiating reprogramming. The release of interleukin-1 receptor antagonists (IL-1RA) induces a shift from a pro-inflammatory stage (dominated by M1 macrophages) to an anti-inflammatory stage (by M2 macrophages). In addition, collagen VII and the structural proteins laminin and keratin 14 are produced, which are also deposited in the wound, aiding its healing3.
The therapeutic approach has already been tested for its efficacy and safety in the course of a phase 1/2 study. Due to the systemic effect via the bloodstream, the stem cells can migrate to the injured tissue sites - to inner (mucous membranes) as well as outer (skin) barrier - implant themselves in the wound and promote its healing.
The multicentre pivotal phase 3 trial aims to enroll 74 patients (children and adults) worldwide. EB House Austria will be the first study centre to enrol patients in the study in the coming weeks.
Rheacell has created a dedicated microsite with clear and patient-friendly information about the trial. This can be viewed via this link: RHEACELL >< EB-Trial
Search our database for active and completed clinical EB trials